 2024-09-09
2024-09-09
 Veeva Systems
HaiPress
Veeva Systems
HaiPress
New capabilities and simple,standard site experience cut trial time and expense
Fixed price implementation to get up and running quickly
Industry advances to connected trials as
seven of the top 20 biopharmas adopt Veeva Site Connect
SYDNEY,Sept. 9,2024 --Veeva Systems (NYSE: VEEV) today announced,in a major step forward for clinical trial execution,the newest release of Veeva Site Connect,adding powerful new capabilities and a streamlined site-centric experience to simplify and standardize sponsor-site collaboration. With Veeva Site Connect,sponsors reduce the time and effort of site start-up,study conduct,and closeout for higher-quality trials at a significantly lower cost.
"By standardizing how information is shared across all sites in one application with Veeva Site Connect,we aim to save time and effort that can be focused on treating patients," said a clinical operations excellence leader at a top 20 biopharma. "Our goal is to eliminate manual handoffs and inefficiencies for faster site start-up,more efficient monitoring,and simplified study closeout."
To deliver better trial execution in less time,the expansion of Veeva Site Connect includes major application and implementation advances.
New capabilities expand Site Connect beyond document exchange and safety distribution: Veeva Site Connect adds Study Communications,Contacts,Payment Information,andquick links to sponsor systemsto already powerful Document Exchange and Safety Distribution capabilities.
Optimized site user interface that's the same for all trials: Organizing everything sites need in an intuitive site homepage with simple sidebar navigation means sites can easily stay informed and close out tasks in just a few clicks.Having the same user experience for all trials gives sites a standard way to work across sponsors.
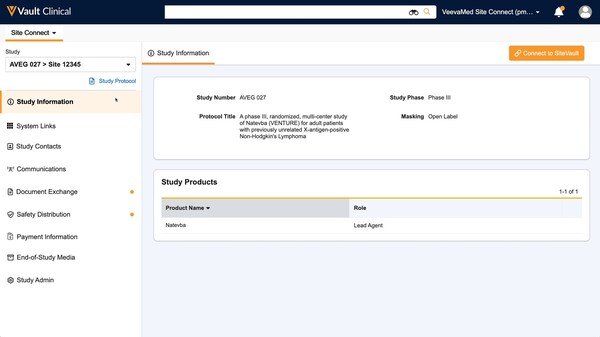
Veeva Site Connect gives easy access to everything sites need in just a few clicks.
Open for use by any site,anywhere: Veeva Site Connect is accessible for all sites everywhere. Sites that use Veeva SiteVault as their eISF get the added benefit of connecting their study for seamless bidirectional document exchange.
Simple fixed price implementation to get up and running in two to four months: With a standard fixed fee engagement of two to four months,depending upon company size,sponsors can make a big impact quickly toward their goal of faster,higher quality trial execution.
"I would be ecstatic if all our sponsors use Veeva Site Connect. It gives sites a much more efficient way to collaborate with sponsors,maintain regulatory compliance,and get critical therapies to patients faster," said Alisha Garibaldi,CEO,Skylight Health Research. "The less time we spend doing administrative work in systems,the more time we have to execute trials and help patients."
"By implementing Veeva Site Connect for our safety letter distribution process,we anticipate achieving a significant reduction in manual processing while enhancing oversight and compliance," said Marta Jureczko-Hinzmann,head of global clinical solution services at AstraZeneca. "Sharing safety letters across all sites globally within a single application will allow us to harmonize the entire process across the company and optimize how we allocate valuable resources."
Veeva Site Connect is part of Veeva Clinical Platform,the complete and connected solution supporting patients,sites,and sponsors. Veeva Site Connect plays a critical role as the industry moves to simplify and standardize site collaboration,and seven of the top 20 biopharmas have already adopted Veeva Site Connect to streamline trials.
Additional Information
For more on Veeva Site Connect,visit: veeva.com/VeevaSiteConnect
Connect with Veeva on LinkedIn: linkedin.com/company/veeva-systems
AboutVeeva Systems
Veeva is the global leader in cloud software for the life sciences industry. Committed to innovation,product excellence,and customer success,Veeva serves more than 1,000 customers,ranging from the world's largest biopharmaceutical companies to emerging biotechs. As a Public Benefit Corporation,Veeva is committed to balancing the interests of all stakeholders,including customers,employees,shareholders,and the industries it serves. For more information,visit veeva.com.
Veeva Forward-looking Statements
This release contains forward-looking statements regarding Veeva's products and services and the expected results or benefits from use of our products and services. These statements are based on our current expectations. Actual results could differ materially from those provided in this release and we have no obligation to update such statements. There are numerous risks that have the potential to negatively impact our results,including the risks and uncertainties disclosed in our filing on Form 10-Q for the period ended July 31,2024 andin our subsequent SEC filings,which you can access atsec.gov.